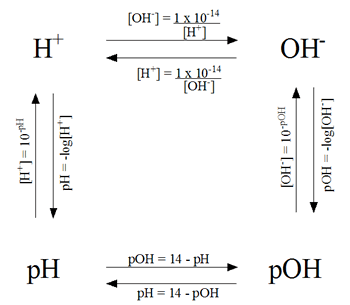
Calculations of pH, pOH, [H+] and [OH-]
pH Problem Solving Diagram
The pH of a 0.0001-M solution of NaOH is:
- 4.0
- -4.0
- 11.0
- 10.0
What is the [OH-] of a solution whose [H+] = 0.001M
- 1 x 10-3 M
- 1 x 10-11 M
- 1 x 1011 M
- 1 x 103 M
The [H+] of a solution is 8.34 x 10-5 mole/liter. The pH of this solution lies between:
- 2 and 3
- 3 and 4
- 4 and 5
- 5 and 6
Which of the following hydrogen ion concentrations represents a solution with acidic properties?
- 1 x 10-2 M
- 1 x 10-8 M
- 1 x 10-10 M
- 1 x 10-14 M
The [H+] of an acid solution that has a pH of 3.0 is:
- 1 x 103 M
- 1 x 10-3 M
- 1 x 1011 M
- 1 x 10-11 M
The pH of a 0.01-M solution of HCl is:
- 2.0
- -2.0
- 12.0
- -12.0
The pH of a solution is 3.0. What is the [OH-]?
- 11 M
- 3 M
- 1 x 10-3 M
- 1 x 10-11 M
What is the pH of a 0.001 M NaOH solution?
- 3.0
- -3.0
- 10.0
- 11.0
The pH of a softdrink is determined to be 4.0. What is the [OH-] of the drink?
- 10 M
- 4 M
- 1 x 10-4 M
- 1 x 10-10 M
In aqueous solutions, [H+][OH-] is equal to:
- 1 x 10-7 M
- 1 x 10-14 M
- 7 M
- 1 x 1014 M
The normal pH of human blood is 7.4. Human blood is:
- slightly basic
- slightly acidic
- strongly basic
- strongly acidic
What is the pOH of a solution whose pH is 3.45?
- -3.45
- 17.45
- 10.55
- 3.45
What is the pH of a solution whose [H+] is 2.75 x 10-4 M?
- 3.561
- 3.636 x 10-11
- 3.64
- 10.439
What is the pOH of a solution whose [H+] is 2.75 x 10-4 M?
- 3.561
- 3.636 x 10-11
- 3.64
- 10.439
What is the pOH of a solution whose [OH-] is 9.31 x 10-2 M?
- 1.031
- 12.969
- 1.07 x 10-13
- 15.031
What is the pH of a solution whose [OH-] is 9.31 x 10-2 M?
- 1.031
- 12.969
- 1.07 x 10-13
- 15.031
What is the [H+] of a solution whose [OH-] is 9.31 x 10-2 M?
- 9.31 x 10-16 M
- 9.31 x 1012 M
- 1.07 x 10-13 M
- 10.44
What is the pH of a solution whose pOH is 11.09?
- 2.91
- 15.09
- 25.09
- -11.09
What is the [H+] of a solution whose pH = 5.43 ?
- 3.7 x 10-6 M
- 2.7 x 10-9 M
- 8.6
- 269153
What is the [OH-] of a solution whose pH = 5.43 ?
- 3.7 x 10-6 M
- 2.7 x 10-9 M
- 8.6
- 269153
What is the [OH-] of a solution whose pOH = 2.86 ?
- 1.4 x 10-3 M
- 7.2 x 10-12 M
- 724 M
- 3.5 x 10-15 M
What is the [H+] of a solution whose pOH = 2.86 ?
- 1.4 x 10-3 M
- 7.2 x 10-12 M
- 724 M
- 3.50 x 10-15 M