- Gas pressure is caused by:
- barometers
- gas molecules colliding with surfaces
- gas molecules condensing to a liquid
- gas molecules hitting other gas molecules
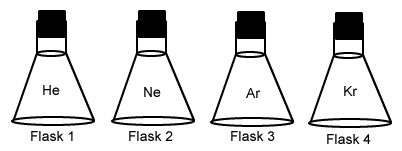
- Which of the following gases would have the fastest rate of diffusion, assuming all of the gases are at the same temperature?
- He
- Ne
- Ar
- Kr
- Which of the following gases would have the slowest rate of diffusion, assuming all of the gases are at the same temperature?
- He
- Ne
- Ar
- Kr
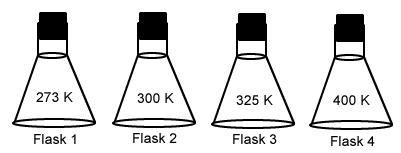
Each of these flasks contains the same number of gas molecules. In which would the pressure be highest?- Flask 1
- Flask 2
- Flask 3
- Flask 4

Each of these flasks contains the same number of gas molecules. In which would the pressure be lowest?- Flask 1
- Flask 2
- Flask 3
- Flask 4
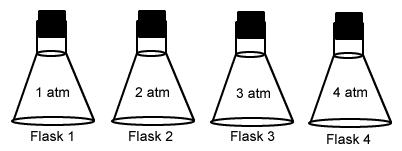
If all of the following flasks are the same size, at the same temperature, and contain the same number of molecules, in which flask will the pressure be highest?- Flask 1
- Flask 2
- Flask 3
- Flask 4
- All have the same pressure

If all of the following flasks are the same size, at the same temperature, and contain the same number of molecules, in which flask will the molecules be moving fastest?- Flask 1
- Flask 2
- Flask 3
- Flask 4

If all of the following flasks are the same size, at the same temperature, and contain the same number of molecules, in which flask will the molecules be moving slowest?- Flask 1
- Flask 2
- Flask 3
- Flask 4
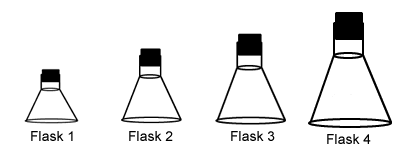
Each of these flasks is the same size and at the same temperature. Which one contains the most molecules?- Flask 1
- Flask 2
- Flask 3
- Flask 4

Each of these flasks is the same size and at the same temperature. Which one contains the fewest molecules?- Flask 1
- Flask 2
- Flask 3
- Flask 4

Each of these flasks is the same size and at the same temperature. In which flask will diffusion occur most slowly?- Flask 1
- Flask 2
- Flask 3
- Flask 4
- Four different flasks contain gas samples at 298 K. In which of the following samples will the gas molecules be moving the fastest?
- H2
- O2
- N2
- CO2
- Which of the following changes to a system WILL NOT result in an increase in pressure?
- Decreasing the volume of the container
- Adding more gas molecules
- Raising the temperature
- Increasing the volume of the container
Each of these flasks contains the same number of molecules. In which container is the pressure highest?- Flask 1
- Flask 2
- Flask 3
- Flask 4
- Four different flasks contain gas samples at 298 K. In which of the following samples will the gas molecules be moving the slowest?
- H2
- O2
- N2
- CO2

Each of these flasks contains the same number of molecules. In which container is the pressure lowest?- Flask 1
- Flask 2
- Flask 3
- Flask 4