- The substance dissolved in a solution is the:
- solvent
- solution
- solute
- mixture
- The dissolving medium in a solution is the:
- solvent
- solution
- solute
- mixture
- A homogeneous mixture of two or more substances is a:
- solvent
- solution
- solute
- compound
- In a solution of sugar and water:
- the sugar is the solute, and the water is the solvent
- the water is the solute, and sugar is the solvent
- both the sugar and the water are solvents
- both the sugar and the water are solutes
- In a solution of ammonia gas in water:
- the ammonia is the solute and water is the solvent
- the water is the solute and ammonia is the solvent
- both the ammonia and the water are solvents
- both the ammonia and the water are solutes
- Naphthalene, a non-polar solid can be dissolved in benzene, a non-polar liquid. From this information, one can conclude that
- benzene is the solute, and naphthalene is the solvent
- naphthalene is the solute, and benzene is the solvent
- naphthalene and benzene are both solvents
- naphthalene and benzene are both solutes
- Of the following, which will increase the solubility of a gas in water?
- increasing the temperature and the increasing the pressure
- decreasing the temperature and increasing the pressure
- decreasing the temperature and decreasing the pressure
- increasing the temperature and decreasing the pressure
- Increasing the temperature of a solution:
- increases the frequency of solute - solvent collisions, but decreases the energy of solute - solvent collisions
- decreases the frequency of solute - solvent collisions, but increases the energy of solute - solvent collisions
- increases both the frequency and the energy of solute - solvent collisions
- decreases both the frequency and the energy of solute - solvent collisions
- The speed of solvent molecules can be slowed by:
- Increasing the surface area of the solute
- Increasing the temperature
- Increasing the pressure
- Decreasing the temperature
- Which of the following should most greatly increase the rate of dissolving of a salt in water?
- Increasing the temperature and stirring
- Increasing the pressure
- Decreasing the temperature and stirring
- Stirring and increasing the pressure
- When a solid or gas dissolves easily in a liquid to form a solution, the solid or gas is said to be _________________ in the liquid.
- compatible
- soluble
- heterogeneous
- endothermic
- A substance that dissolves in a water to form a solution that conducts an electric current is said to be an:
- electrolyte
- nonelectrolyte
- heterogeneous solvent
- catalyst
- Which of the following is an example of a nonelectrolyte in water solution?
- base
- sugar
- salt
- acid
- Which of the following will conduct an electric current?
- sugar in water
- alcohol in water
- salt in water
- pure water
- Breaking a solid into smaller pieces increases the rate of dissolving because
- it increases the temperature of the solution
- it increases the energy of the solution
- it increases the surface area of the solute
- it increases the pressure above the solution
- Which of the following is least likely to produce a solution?
- An ionic solute in a polar solvent
- A polar solute in a polar solvent
- A nonpolar solute in a nonpolar solvent
- A nonpolar solute in a polar solvent
- When a solute dissolves in water, it is expected to
- raise the freezing point and the boiling point of the water
- lower the freezing point and the boiling point of the water
- raise the freezing point and lower the boiling point of the water
- lower the freezing point and raise the boiling point of the water
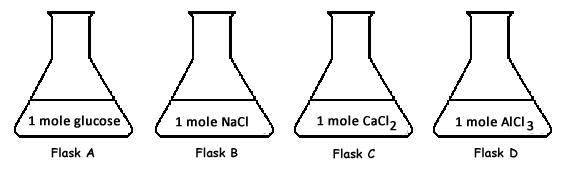
Assume that the image above represents the given quantity of each substance dissolved in one liter of water. Which of the following statements is true?- All of the solutions will boil at a temperature below 100°C
- All of the solutions will boil at 100°C
- Some solutions will boil at a temperature below 100°C, and some of the solutions will boil at a temperature above 100°C
- All of the solutions will boil at a temperature above 100°C

Assume that the image above represents the given quantity of each substance dissolved in one liter of water. Which of the following statements is true?- All of the solutions will freeze at a temperature below 0°C
- All of the solutions will freeze at 0°C
- Some solutions will freeze at a temperature below 0°C, and some of the solutions will freeze at a temperature above 0°C
- All of the solutions will freeze at a temperature above 0°C
- When two liquids blend together to form a solution, the liquids are said to be
- co-soluble
- soluble in one another
- miscible
- nonpolar solutes