- At room conditions, the density of liquid water is about
- 1 g/mL
- 18 g/ml
- 22.4 g/mL
- 0 g/mL
- The boiling point of pure water at standard pressure is
- 0°C
- 32°C
- 100°C
- 212°C
- The freezing point of pure water at standard pressure is
- 0°C
- 32°C
- 100°C
- 212°C
- Which of these phase changes occurs by the removal of energy?
- Which of these phase changes occurs by the absorption of energy?
- Separate 100 gram masses of iron and liquid water are each allowed to absorb 600 joules of energy. As a result,
- the temperature of the water changes more than the temperature of the iron
- the temperature of the iron changes more than the temperature of the water
- the temperatures of both change by the same amount
- neither the water nor the iron experience a temperature change
- The temperature would be expected to remain constant during which of the following phase changes?
- Use this information to answer the question below:
ΔHfus (H2O) = 334 J/g
ΔHvap (H2O) = 2260 J/g
How much energy is required to boil 50 grams of water at its boiling point?- 100 J
- 113 000 J
- 45.2 J
- 50 J
- Use this information to answer the question below:
ΔHfus (H2O) = 334 J/g
ΔHvap (H2O) = 2260 J/g
How much energy is required to melt 50 grams of ice at its melting point?- 16 700 J
- 113 000 J
- 6.68 J
- 50 J
- Which of these has the greatest density, assuming standard pressure?
- Liquid water at 4°C
- Liquid water at 95°C
- Ice at -10°C
- Steam at 120°C
- Refer to the image below when answering this question. Assume standard pressure.
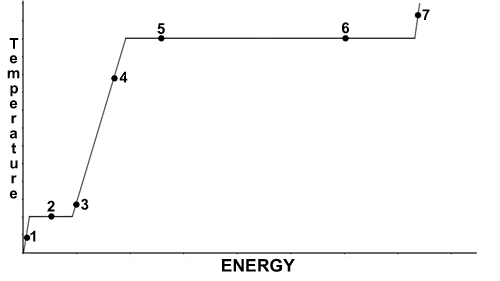
As energy is added and one moves from point #5 to point #6, the change taking place is- an increase in the temperature of liquid water
- an increase in the density of water
- liquid water is becoming steam
- ice is melting
- Refer to the image below when answering this question. Assume standard pressure.

As energy is added and one moves from point #5 to point #6, the temperature- remains constant
- increases
- decreases
- Refer to the image below when answering this question. Assume standard pressure.

The temperature at point #6 is- 100°C
- 32°C
- 0°C
- 100°F
- Refer to the image below when answering this question. Assume standard pressure.

At point #2, water would be found as- a liquid only
- a solid only
- a solid and a liquid
- a liquid and a vapor
- a vapor only
- Refer to the image below when answering this question. Assume standard pressure.

As energy is being removed (going right to left), the phase change occuring at point #2 would be- freezing
- boiling
- condensation
- melting
- Refer to the image below when answering this question. Assume standard pressure.

The temperature at point #1 is- below 0°C
- 0°C
- Between 0°C and 100°C
- above 100°C
- Refer to the image below when answering this question. Assume standard pressure.

The density of water is highest at point- 1
- 2
- 3
- 4
- 5
- 6
- 7
- Refer to the image below when answering this question. Assume standard pressure.

The measure of the energy required to move from point #3 to point #4 on the graph is determined by the- specific heat of liquid water
- specific heat of steam
- specific heat of ice
- heat of fusion of water
- heat of vaporization of water
- Refer to the image below when answering this question. Assume standard pressure.

Which forms of water would be present at point #7?- liquid only
- steam only
- ice only
- liquid and steam
- solid and liquid
- Refer to the image below when answering this question. Assume standard pressure.

The difference in temperature between point #2 and point #6 is- 100°C
- 273°C
- 32°F
- 212°F
- Refer to the image below when answering this question. Assume standard pressure.

Features of this graph support which observations about water? - Which of these is NOT a characteristic of most pure substances, but is true of water?