1 / 25
- When the temperature is decreased on a closed system containing water and its vapor at equilibrium:H2O(l) + heat ⇌ H2O(g)
- In order to restore equilibrium, more liquid water evaporates
- In order to restore equilibrium, water vapor condenses to form liquid water
- No change occurs
- This is the aqueous iron(III) thiocyanate equilibrium:Fe3+(yellow) + SCN-(colorless) ⇌ [FeSCN]2+(dark red)
If aqueous potassium thiocyanate is added to the solution at equilibrium:- No change in color occurs
- The solution turns darker red
- The solution becomes colorless
- The solution becomes more yellow
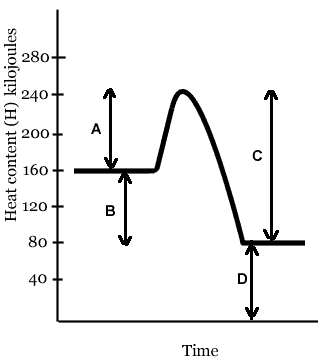
The line that represents the activation energy (Ea) of this reaction is- Line B
- Line C
- Line A
- Line D
The activation energy (Ea) of this reaction is- 240 kJ
- 80 kJ
- 40 kJ
- - 80 kJ
- 160 kJ
- A precipitation reaction achieves equilibrium:BaCl2(aq) + Na2SO4(aq) ⇌ 2 NaCl(aq) + BaSO4(s)
If barium chloride (BaCl2) is added to the system, which change occurs?- The reaction shifts to the left, and some precipitate dissolves
- The reaction shifts to the right, and more precipitate is produced
- No change occurs
- At 25 ºC, a certain reaction is able to produce 0.80 moles of product per minute? At what rate might the product be produced at 35 ºC?
- 0.80 moles per minute
- 0.20 moles per minute
- 1.6 moles per minute
- 0.40 moles per minute

The line that represents the heat of reaction (ΔH, or ΔE) of this reaction is- Line A
- Line C
- Line D
- Line B
- An increase in temperature increases the rate of a chemical reaction by what means
- Given the following reaction:C3H8(g) + 5 O2(g) → 3 CO2
+ 4 H2O(g)
If the intial rate of the reaction produces 2 moles of water vapor, H2O, per second, what is the rate at which oxygen, O2, is consumed initially?- 2.5 moles per second
- 1 mole per second
- 8 moles per second
- 2 moles per second
- When the temperature is decreased on the following system at equilibrium:2 HCl(aq) + Mg(s) ⇌ MgCl2(aq) + H2(g) + heat
- In order to restore equilibrium, the reaction shifts left, toward reactants
- No change occurs
- In order to restore equilibrium, the reaction shifts right, toward products

In the reaction depicted in the diagram above,- the energy content of the products is greater than the energy content of the reactants
- the energy content of the reactants is greater than the energy content of the products
- the energy content of the reactants is the same as the energy content of the products
- The process of dissolving Na2SO4 in water is known to be exothermic:Na2SO4(s) ⇌ 2 Na+(aq) + SO42-(aq) + heat
If the temperature of the solution is decreased, Na2SO4 becomes:- No change in solubility occurs
- More soluble
- Less soluble
- When N2 is removed from the following system at equilibrium:3 H2(g) + N2(g) ⇌ 2 NH3(g)
- No change occurs
- In order to restore equilibrium, the reaction shifts left, toward reactants
- In order to restore equilibrium, the reaction shifts right, toward products

The reaction whose energy diagram is shown here is- isothermic
- exothermic
- endothermic
- Given the following reaction:P4(s) + 5 O2(g) → 2 P2O5(s)
If the intial rate of the reaction consumes 1 mole of phosphorus, P4, per second, what is the rate at which oxygen, O2, is consumed initially?- 0.2 moles per second
- 2 moles per second
- 1 mole per second
- 5 moles per second
- At 25 ºC, a certain reaction is able to produce 0.80 moles of product per minute? At what rate might the product be produced at 15 ºC?
- 1.20 moles per minute
- 1.6 moles per minute
- 0.80 moles per minute
- 0.40 moles per minute
- Given the following reaction:C3H8(g) + 5 O2(g) → 3 CO2
+ 4 H2O(g)
If the intial rate of the reaction produces 2 moles of water vapor, H2O, per second, what is the rate at which propane, C3H8, is consumed initially?- 1 mole per second
- 4 moles per second
- 0.5 moles per second
- 2 moles per second
- When extra NH3 is added to the following system at equilibrium:3 H2(g) + N2(g) ⇌ 2 NH3(g)
- In order to restore equilibrium, the reaction shifts left, toward reactants
- No change occurs
- In order to restore equilibrium, the reaction shifts right, toward products
- When the temperature is increased on the following system at equilibrium:2 HCl(aq) + Mg(s) ⇌ MgCl2(aq) + H2(g) + heat
- In order to restore equilibrium, the reaction shifts left, toward reactants
- In order to restore equilibrium, the reaction shifts right, toward products
- No change occurs
- When the pressure is increased on a closed system containing water and its vapor at equilibrium:H2O(l) + heat ⇌ H2O(g)
- In order to restore equilibrium, more liquid water evaporates
- In order to restore equilibrium, water vapor condenses to form liquid water
- No change occurs
- When the pressure is increased on the following system at equilibrium:3 H2(g) + N2(g) ⇌ 2 NH3(g)
- No change occurs
- In order to restore equilibrium, the reaction shifts right, toward products
- In order to restore equilibrium, the reaction shifts left, toward reactants

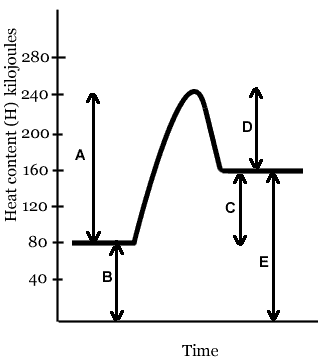
The line that represents the heat of reaction (ΔH, or ΔE) of this reaction is- Line E
- Line D
- Line B
- Line C
- Line A
- A beaker with a mixture of ice and water is maintained at equilibrium:H2O(s) + heat ⇌ H2O(l)
If the temperature of the system is decreased:- No change occurs
- Water freezes to form ice (shift to the left)
- Ice melts to form liquid water (shift to the right)

If you were holding in your hand a test tube in which the reaction above is taking place, it would- feel cold, because energy is being absorbed
- feel hot, because energy is being absorbed
- feel cold, because energy is being released
- feel hot, because energy is being released
- Which of the following will increase the rate of a chemical reaction?