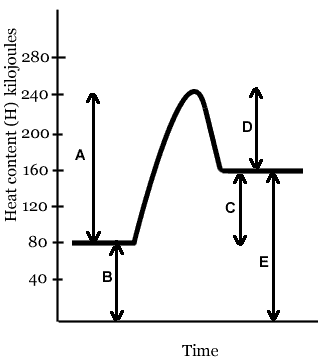
The line that represents the activation energy (Ea) of this reaction is- Line A
- Line B
- Line C
- Line D
- Line E

The line that represents the heat of reaction (ΔH, or ΔE) of this reaction is- Line A
- Line B
- Line C
- Line D
- Line E

The heat of reaction (ΔH, or ΔE) of this reaction is- 80 kJ
- -80 kJ
- 160 kJ
- 240 kJ
- -160 kJ

The activation energy (Ea) of this reaction is- 160 kJ
- 80 kJ
- 240 kJ
- 40 kJ
- - 80 kJ

In the reaction depicted in the diagram above,- the energy content of the reactants is greater than the energy content of the products
- the energy content of the products is greater than the energy content of the reactants
- the energy content of the reactants is the same as the energy content of the products

If you were holding in your hand a test tube in which the reaction above is taking place, it would- feel cold, because energy is being absorbed
- feel hot, because energy is being absorbed
- feel cold, because energy is being released
- feel hot, because energy is being released

If a catalyst were added to this reaction, which lines would change?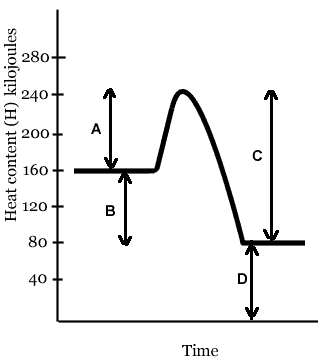
The line that represents the activation energy (Ea) of this reaction is- Line A
- Line B
- Line C
- Line D

The line that represents the heat of reaction (ΔH, or ΔE) of this reaction is- Line A
- Line B
- Line C
- Line D

The heat of reaction (ΔH, or ΔE) of this reaction is- 80 kJ
- -80 kJ
- 160 kJ
- 240 kJ
- -160 kJ

The activation energy (Ea) of this reaction is- 160 kJ
- 80 kJ
- 240 kJ
- 40 kJ
- - 80 kJ

In the reaction depicted in the diagram above,- the energy content of the reactants is greater than the energy content of the products
- the energy content of the products is greater than the energy content of the reactants
- the energy content of the reactants is the same as the energy content of the products

If you were holding in your hand a test tube in which the reaction above is taking place, it would- feel cold, because energy is being absorbed
- feel hot, because energy is being absorbed
- feel cold, because energy is being released
- feel hot, because energy is being released

If a catalyst were added to this reaction, which lines would change?
The reaction whose energy diagram is shown here is- endothermic
- exothermic
- isothermic

The reaction whose energy diagram is shown here is- endothermic
- exothermic
- isothermic