- The specific heat of aluminum is 0.90 J/g·°C. How much energy is required to raise the temperature of 10 grams of aluminum from 10°C to 20°C?
- 90 joules
- 0.009 joules
- 111 joules
- 100.9 joules
- A 100 gram sample of a metal undergoes a temperature change from 20°C to 50° after absorbing 1500 J of heat. What is the specific heat of the metal?
- 0.5 J/g·°C
- 2 J/g·°C
- 4.5 x 106 J/g·°C
- 0.25 J/g·°C
- How many joules of heat energy are required to raise the temperature of 20 grams of water by 50°C?
- 4180 joules
- 239.2 joules
- 0.00418 joules
- 19.7 joules
- What energy change has occured occurred when 500 grams of water cools from 35°C to 25°C?
- -20 900 joules
- 20 900 joules
- -1196 joules
- 11.96 joules
- How many joules are required to vaporize 10 grams of water at its boiling point?
- 22 600 joules
- 226 joules
- 0.0044 joules
- 2270 joules
- How much energy must be removed from 2 grams of water at 0°C to convert the water to ice?
- 668 joules
- 167 joules
- 0.00599 joules
- 336 joules
- What mass of ice at 0°C can be melted by the addition of 1670 joules of heat?
- 5 grams
- 0.2 grams
- 8350 grams
- 1670 grams
- How many grams of water at 100°C can be converted to steam by the addition of 565 joules of heat?
- 0.25 grams
- 4 grams
- 56 500 grams
- 1 276 900 joules
- A 100 gram piece of brass at a temperature of 100°C is dropped into 100 grams of water that has a temperature of 20°C. Which of the following describes what happens next?
- They come to equilibrium at a temperature above 60°C, because the metal has a higher specific heat than does the water.
- They come to equilibrium at a temperature above 60°C, because the metal has a lower specific heat than does the water.
- They come to equilibrium at a temperature below 60°C, because the metal has a higher specific heat than does the water.
- They come to equilibrium at a temperature below 60°C, because the metal has a lower specific heat than does the water.
- When a piece of gold (specific heat 0.13 J/g·°C) and a piece of iron (specific heat 0.46 J/g·°C) each absorb an equal amount of heat
- the gold will end up with the higher final temperature
- the iron will end up with the higher final temperature
- they will end up at the same final temperature
- Which of the following phase changes involve the addition (absorption) of heat?
- Which of the following phase changes involve the removal (release) of heat?
- When you mixed magnesium with hydrochloric acid in the lab, the reaction got warmer. This indicates that
- the reaction is exothermic, because energy was released
- the reaction is endothermic, because energy was released
- the reaction is exothermic, because energy was absorbed
- the reaction is endothermic, because energy was absorbed
- When you mixed baking soda with citric acid in the lab, the reaction got colder. This indicates that
- the reaction is exothermic, because energy was released
- the reaction is endothermic, because energy was released
- the reaction is exothermic, because energy was absorbed
- the reaction is endothermic, because energy was absorbed
- In an exothermic reaction,
- the energy content of the reactants is greater than the energy content of the products
- the energy content of the reactants is less than the energy content of the products
- the energy content of the reactants is the same as the energy content of the products
- In an endothermic reaction,
- the energy content of the reactants is greater than the energy content of the products
- the energy content of the reactants is less than the energy content of the products
- the energy content of the reactants is the same as the energy content of the products
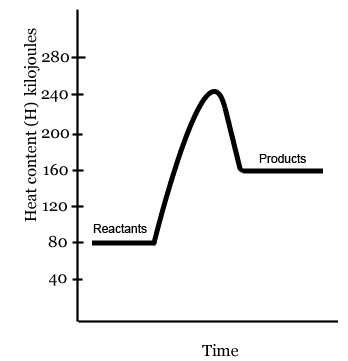
In the diagram above, the energy content of the reactants is- 80 kilojoules
- 160 kilojoules
- 240 kilojoules

In the diagram above, the energy content of the products is- 80 kilojoules
- 160 kilojoules
- 240 kilojoules

In the diagram above, the activation energy (Ea) would be calculated by doing which of the following?- 240 kilojoules - 160 kilojoules = 80 kilojoules
- 240 kilojoules - 80 kilojoules = 160 kilojoules
- 80 kilojoules + 160 kilojoules = 240 kilojoules
- 160 kilojoules - 80 kilojoules = 80 kilojoules

In the diagram above, the heat of reaction (ΔH) would be calculated by doing which of the following?- 240 kilojoules - 160 kilojoules = 80 kilojoules
- 240 kilojoules - 80 kilojoules = 160 kilojoules
- 240 kilojoules + 160 kilojoules = 240 kilojoules
- 160 kilojoules - 80 kilojoules = 80 kilojoules

In the diagram above, the energy content of the products- is the same as the energy content of the reactants
- is the greater than the energy content of the reactants
- is less than the energy content of the reactants

The diagram above depicts a reaction that is- endothermic
- exothermic
- isothermic