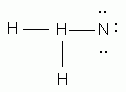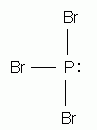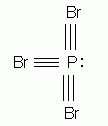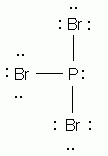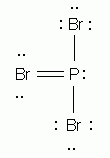Valence electrons are
- unpaired electrons
- inner-shell electrons
- outer-shell electrons
- electrons in the nucleus
Covalent bonds are formed by
- losing electrons
- gaining electrons
- splitting electrons
- sharing electrons
Covalent bonding involves bonds between:
- metals and metals
- metals and nonmetals
- nonmetals and nonmetals
A bond between nitrogen (atomic #7) and oxygen (atomic #8) would be:
- ionic
- covalent
- metallic
The bond between boron (atomic #5) and silicon (atomic #14) is:
- ionic
- covalent
- metallic
The bond in between sodium (atomic #11) and oxygen (atomic #8) is:
- ionic
- covalent
- metallic
The bond between hydrogen (atomic #1) and oxygen (atomic #8) is:
- ionic
- covalent
- metallic
The bond between lithium (atomic #3) and fluorine (atomic #9) is:
- ionic
- covalent
- metallic
A bond between potassium (atomic #19) and chlorine (atomic #17) would be:
- ionic
- covalent
- metallic
The bond in between an oxygen atom and another oxygen atom is:
- ionic
- covalent
- metallic
Which of the following IS NOT a covalent compound?
- CaO
- CO2
- CH3Cl
- NH3
Which of the following IS NOT an ionic compound?
- KCl
- MnO2
- Mg3N2
- C2H5OH
The octet rule explains the stability of most covalently bonded molecules in terms of:
- electronegativity differences
- noble-gas configurations
- ionization energies
- electron affinities
Which of the following elements has the same Lewis dot structure as phosphorus?
- nitrogen
- sulfur
- silicon
- argon
Which of the diatomic elements has a triple bond between its atoms?
- hydrogen
- nitrogen
- oxygen
- fluorine
According to the HONC rule, how many covalent bonds form around hydrogen and the halogens?
According to the HONC rule, how many covalent bonds form around carbon?
According to the HONC rule, how many covalent bonds form around oxygen?
According to the HONC rule, how many covalent bonds form around nitrogen?
Which of the following is an acceptable Lewis Structure for the diatomic nitrogen molecule?
-
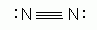
-
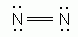
-
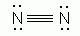
-
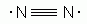
In the correct Lewis structure for the methane (CH4) molecule, how many unshared electron pairs surround the carbon?
- 0
- 2
- 4
- 8
In the correct Lewis structure for water, how many unshared pairs of electrons will oxygen have?
- 1
- 2
- 3
- 4
Which of the following is an acceptable Lewis structure for chloromethane (CH3Cl)?
-
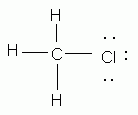
-
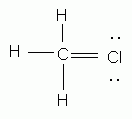
-
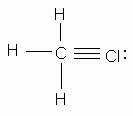
-
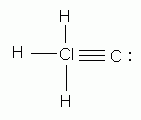
Which of the diatomic elements has a double bond between its atoms?
- hydrogen
- nitrogen
- oxygen
- fluorine
The seven elements that occur as diatomic elements are:
- H2, N2, O2, He2, Ne2, Cl2, Br2
- H2, N2, O2, He2, Ne2, C2, Na2
- H2, N2, O2, F2, Cl2, Br2, I2
- Fe2, Rn2, O2, He2, Ne2, C2, Br2
Which of the following is a correct Lewis structure for hydrogen cyanide, HCN?
-
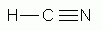
-
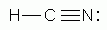
-
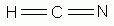
-
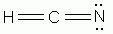
-
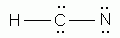
Which of the following is the correct Lewis structure for formaldehyde, CH2O?
-
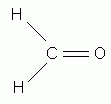
-
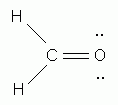
-
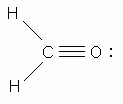
-
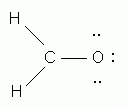
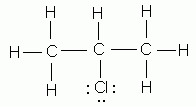
Which of the following is NOT a valid Lewis structure?
-
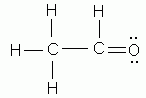
-
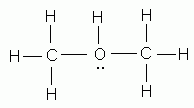
-
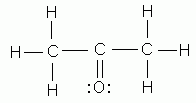
-
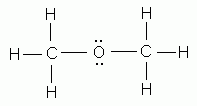
-
Which of the following is the correct Lewis structure for ammonia, NH3?
-
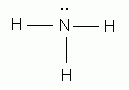
-
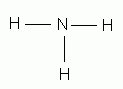
-
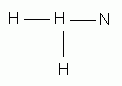
-
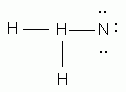
Which of the following is the correct Lewis structure for methanol, CH4O?
-
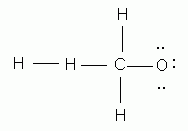
-
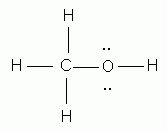
-
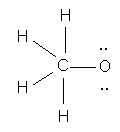
-
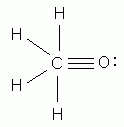
Which of the following is the correct Lewis structure for phosphorus tribromide, PBr3?
-
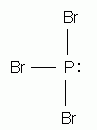
-
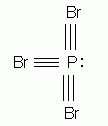
-
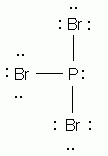
-
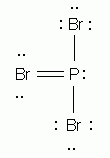
Which of the following is the correct Lewis structure for ethene (ethylene), C2H4?
-
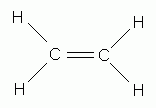
-
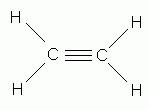
-
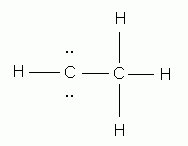
-
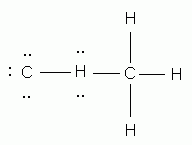
Which of the following elements will NOT be surrounded by an octet of electrons in a correctly drawn Lewis structure?
- Hydrogen
- Carbon
- Chlorine
- Oxygen
In drawing Lewis structures, a single line (single bond) between two elements represents:
- an unshared pair of electrons
- a shared pair of electrons
- an octet of electrons
- a shared electron