Generally speaking, the group of elements with the highest first ionization energy is:
- Group 1
- Group 16
- Group 17
- Group 18
The most active metals are located in the:
- lower right hand corner of the periodic table
- lower left hand corner of the periodic table
- upper right hand corner of the periodic table
- upper left hand corner of the periodic table
The least electronegative elements are the:
- Noble gases
- Alkali metals
- Halogens
- Metalloids
The elements with the largest atomic radii are found in the:
- upper right-hand corner of the periodic table
- upper left-hand corner of the periodic table
- lower right-hand corner of the periodic table
- lower left-hand corner of the periodic table
The elements with the smallest atomic radii are found in the:
- upper right-hand corner of the periodic table
- upper left-hand corner of the periodic table
- lower right-hand corner of the periodic table
- lower left-hand corner of the periodic table
The measure of the attraction that an atom has for electrons involved in chemical bonds is known as:
- electron affinity
- electronegativity
- radioactivity
- ionization energy
The energy required to remove an electron from an atom is known as:
- electron affinity
- electronegativity
- radioactivity
- ionization energy
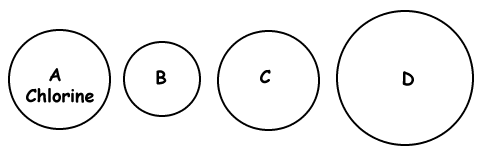
Given the representation of a chlorine atom, which circle might represent an atom of argon?
- None of these
- Circle B
- Circle C
- Circle D

Given the representation of a chlorine atom, which circle might represent an atom of sulfur?
- None of these
- Circle B
- Circle C
- Circle D

Given the representation of a chlorine atom, which circle might represent an atom of fluorine?
- None of these
- Circle B
- Circle C
- Circle D

Given the representation of a chlorine atom, which circle might represent an atom of bromine?
- None of these
- Circle B
- Circle C
- Circle D

Given the representation of a chlorine atom, which circle might a chloride ion, Cl
-?
- None of these
- Circle B
- Circle C
- Circle D
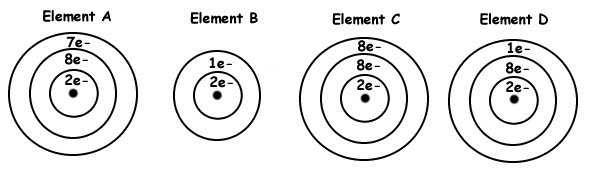
Which of these elements would have the lowest first ionization energy?
- Element A
- Element B
- Element C
- Element D
Cations have a ______________ charge and are ______________ than the atoms from which they formed.
- positive/larger
- positive/smaller
- negative/larger
- negative/smaller
Anions have a ______________ charge and are ______________ than the atoms from which they formed.
- positive/larger
- positive/smaller
- negative/larger
- negative/smaller
As one moves from left to right ( → ) within a period across the periodic table, the atomic radius of the elements encountered tends to:
- increase
- decrease
- stay the same
As one moves from left to right ( → ) within a period across the periodic table, the ionization energy of the elements encountered tends to:
- increase
- decrease
- stay the same
As one moves from left to right ( → ) within a period across the periodic table, the electronegativity of the elements encountered tends to:
- increase
- decrease
- stay the same
As one moves from down ( ↓ ) a group on the periodic table, the electronegativity of the elements encountered tends to:
- increase
- decrease
- stay the same
As one moves from down ( ↓ ) a group on the periodic table, the ionization energy of the elements encountered tends to:
- increase
- decrease
- stay the same
As one moves from down ( ↓ ) a group on the periodic table, the atomic radius of the elements encountered tends to:
- increase
- decrease
- stay the same
Of the following elements, which one would have the largest radius?
- Hydrogen (H, atomic #1)
- Sodium (Na, atomic #11)
- Potassium (K, atomic #19)
- Cesium (Cs, atomic #55)
Of the following elements, which one would have the largest ionization energy?
- Hydrogen (H, atomic #1)
- Sodium (Na, atomic #11)
- Potassium (K, atomic #19)
- Cesium (Cs, atomic #55)
Of the following elements, which one would have the largest electronegativity energy?
- Fluorine (F, atomic #9)
- Chlorine (Cl, atomic #17)
- Bromine (Br, atomic #35)
- Iodine (I, atomic #53)
Of the following elements, which one would have the smallest radius?
- Fluorine (F, atomic #9)
- Chlorine (Cl, atomic #17)
- Bromine (Br, atomic #35)
- Iodine (I, atomic #53)
Of the following elements, which one would have the smallest radius?
- Lithium (Li, atomic #3)
- Boron (B, atomic #5)
- Nitrogen (N, atomic #7)
- Neon (Ne, atomic #10)
Of the following elements, which one would have the smallest ionization energy?
- Lithium (Li, atomic #3)
- Boron (B, atomic #5)
- Nitrogen (N, atomic #7)
- Neon (Ne, atomic #10)
Of the following elements, which one would have the largest radius?
- Lithium (Li, atomic #3)
- Boron (B, atomic #5)
- Nitrogen (N, atomic #7)
- Neon (Ne, atomic #10)
A horizontal row ( → ) of elements on the periodic table may also be referred to as a:
A vertical column ( ↓ ) of elements on the periodic table may also be referred to as a:





