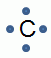
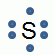- A group of elements that all have six valence electrons:
- Group 1
- Group 16
- Group 17
- Group 18
- A group of elements that all have seven valence electrons:
- halogens
- Noble gases
- transition metals
- alkaline earth metals
- The alkali metals form ions with a charge of
- 2-
- 1-
- 2+
- 1+
- The alkaline earth metals form ions with a charge of
- 2-
- 1-
- 2+
- 1+
- The halogens form ions with a charge of
- 2+
- 1+
- 2-
- 1-
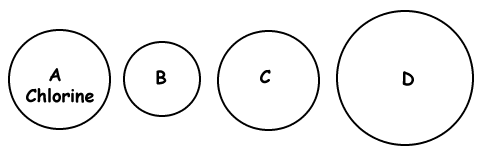
Given the representation of a chlorine atom, which circle might represent an atom of bromine?- None of these
- Circle B
- Circle C
- Circle D
- Which family of elements does not readily form ions?
- Alkali metals
- Alkaline earth metals
- Halogens
- Noble gases
- The nonmetal members of the oxygen family (oxygen, sulfur and selenium) for ions with a charge of
- 2-
- 1-
- 1+
- 2+
- The nonmetal members of the nitrogen family (nitrogen, phosphorus, and arsenic) form ions with a charge of
- 3-
- 2-
- 1-
- 3+
- Which of the following elements would have five dots in its electron dot notation?
- oxygen
- fluorine
- nitrogen
- aluminum
- As one moves down ( ↓ ) a group on the periodic table, the atomic radius of the elements encountered:
- increases
- decreases
- stays the same
- Of the following elements, which one would have the largest radius?
- Hydrogen (H, atomic #1)
- Sodium (Na, atomic #11)
- Potassium (K, atomic #19)
- Cesium (Cs, atomic #55)
- Of the following elements, which one would have the largest radius?
- Fluorine (F, atomic #9)
- Chlorine (Cl, atomic #17)
- Bromine (Br, atomic #35)
- Iodine (I, atomic #53)
- Of the following elements, which one would have the smallest radius?
- Fluorine (F, atomic #9)
- Chlorine (Cl, atomic #17)
- Bromine (Br, atomic #35)
- Iodine (I, atomic #53)
- Which of the following sets of elements could replace the generic "X" symbol with their own to produce a valid dot notation for the element(s)?

- boron, aluminum and gallium
- carbon, silicon and germanium
- nitrogen, phosphorus and arsenic
- oxygen, sulfur and selenium
- Which of the following sets of elements could replace the generic "X" symbol with their own to produce a valid dot notation for the element(s)?

- boron, aluminum and gallium
- carbon, silicon and germanium
- nitrogen, phosphorus and arsenic
- oxygen, sulfur and selenium
- Which of the following is the correct dot notation for the element carbon?
-
- Which of the following is the correct dot notation for the element potassium, K?
-
- Which of the following is the correct dot notation for the element sulfur?
-
- Which of the following sets of elements could replace the generic "X" symbol with their own to produce a valid dot notation for the element(s)?

- Which of the following elements has the same number of valence electrons as the element carbon, C?
- boron, B, atomic #5
- nitrogen, N, atomic #7
- silicon, Si, atomic #14
- phosphorus, P, atomic #15
- Which of these elements does not have the same number of valence electrons as the other three?
- helium
- neon
- argon
- krypton
- Which of these elements does not have the same number of valence electrons as the other three?
- beryllium, Be
- magnesium, Mg
- cesium, Cs
- strontium, Sr
- Which of these is the proper formula for a magnesium ion?
- Mg1+
- Mg2+
- Mg1-
- Mg2-
- When forming ions, metals tend to _________ electrons and nonmetals tend to __________ electrons.
- lose / gain
- gain / lose
- gain / gain
- lose / lose