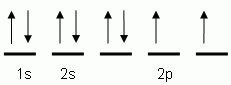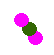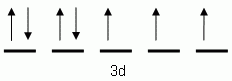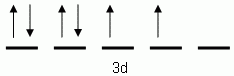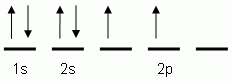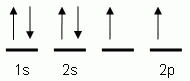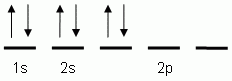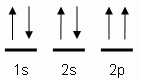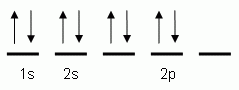The nucleus of most atoms is made up of:
- protons and electrons
- protons and neutrons
- neutrons and electrons
- electrons and protons
The laws of electrostatics consistently demonstrate that opposite charges:
- destroy one another
- attract one another
- repel on another
- ignore one another
The charge and mass number of a proton are:
- charge = +1, Mass number = 0
- charge = -1, Mass number = 0
- charge = 0, Mass number = 1
- charge = +1, Mass number = 1
The charge and mass number of an electron are:
- charge = +1, Mass number = 0
- charge = -1, Mass number = 0
- charge = 0, Mass number = 1
- charge = +1, Mass number = 1
The charge and mass number of a neutron are:
- charge = +1, Mass number = 0
- charge = -1, Mass number = 0
- charge = 0, Mass number = 1
- charge = +1, Mass number = 1
Most of the mass of the atom can be found in the:
- electron cloud
- nucleus
- electrons
- charges
Which of the following descriptions does not apply to the nucleus?
- Very dense
- Mostly empty space
- Positively charged
- Very small
Of the basic atomic particles, the one that would be attracted to a negatively charged metallic plate is the:
- proton
- neutron
- electron
The mass number of an atom is determined by:
- adding the protons and electrons
- adding the neutrons and electrons
- adding the neutrons and protons
- the number of protons only
The _______________ constitute(s) most of the volume of an atom.
- protons
- neutrons
- nucleus
- electron cloud
The atomic number of an element is equal to:
- the number of protons in the atom
- the number of neutrons in the atom
- the number of protons plus the number of neutrons
- the number of protons plus the number of electrons
How many electrons, neutrons and protons would be found in an atom of carbon-14 (atomic number 6)?
- 6 electrons, 6 neutrons, 6 protons
- 6 electrons, 6 neutrons, 8 protons
- 8 electrons, 8 neutrons, 6 protons
- 6 electrons, 8 neutrons, 6 protons
How many electrons would be found in an atom of oxygen (atomic number 8)?
- 2
- 4
- 6
- 8
An element with nine protons in every atom must:
- have nine neutrons as well
- be fluorine
- have a mass number of nine
- be unstable
There are three isotopes of the element argon: argon-36, argon-38 and argon-40. These isotopes differ from one another in:
How many neutrons are there in an atom of hydrogen-3?
- 0
- 1
- 2
- 3
| Isotope | Mass number | Atomic number | Protons | Neutrons | Electrons |
| Scandium-45 | | | | | |
- The blanks, respectively, would be filled: 21, 45, 21, 21, 24
- The blanks, respectively, would be filled: cobalt, 40, 27, 13, 27
- The blanks, respectively, would be filled: aluminum, 27, 13, 14, 13
- The blanks, respectively, would be filled: silicon, 27, 14, 13, 13
| Isotope | Mass number | Atomic number | Protons | Neutrons | Electrons |
| _______________-27 | | | | | 13 |
- The blanks, respectively, would be filled: aluminum, 27, 13, 13, 14
- The blanks, respectively, would be filled: cobalt, 40, 27, 13, 27
- The blanks, respectively, would be filled: aluminum, 27, 13, 14, 13
- The blanks, respectively, would be filled: silicon, 27, 14, 13, 13
A process in which a very heavy nucleus splits into more-stable nuclei of intermediate mass.
- nuclear fusion
- nuclear fission
- radioactive decay
- a chain reaction
- radiocarbon dating
An electron emitted from the nucleus during some kinds of radioactive decay is known as:
- An alpha particle
- A beta particle
- A positron
- A gamma ray
An alpha particle is essentially a ____________________ nucleus.
- hydrogen
- uranium
- carbon-12
- helium
- plutonium
Gamma rays are:
- neutrons
- protons
- electrons
- pure energy waves
Very large nuclei tend to be unstable because of the:
- repulsive forces between electrons
- attraction of electrons for the positively charged nucleus
- repulsive forces between neutrons
- repulsive forces between protons
- attraction of protons for neutrons
For the most common types of radioactive decay, the order of mass from heaviest to lightest is:
- gamma, alpha, beta
- beta, alpha, gamma
- beta, gamma, alpha
- alpha, beta, gamma
For the most common types of radioactive decay, the order of least penetrating to human tissue, to most penetrating to human tissue is:
- gamma, alpha, beta
- alpha, beta, gamma
- beta, gamma, alpha
- gamma, beta, alpha
For the most common types of radioactive decay, the order of least dangerous to most dangerous is:
- gamma, alpha, beta
- alpha, beta, gamma
- beta, gamma, alpha
- gamma, beta, alpha
In nuclear reactions:
- small amount of mass are converted to large amounts of mass
- large amount of energy are converted to small amount of mass
- small amounts of mass are converted to large amount of energy
- mass and energy are destroyed
Compared to chemical reactions, nuclear reactions produce:
- proportionally far less energy
- proportionally far more energy
- fewer changes in the nucleus
- more vegetables
Which of the following statements is true?
- All man-made isotopes are radioactive
- Some man-made isotopes are radioactive
- None of the man-made isotopes are radioactive
Which of the following statements is true?
- All naturally occurring isotopes are radioactive
- Some naturally occurring isotopes are radioactive
- No naturally occurring isotopes are radioactive

If we assume that pink represents protons, and green represents neutrons, the nucleus depicted here is:
- 76C
- 613C
- 713C
- 136C

If we assume that pink represents protons, and green represents neutrons, the nucleus depicted here is:
- boron-6
- boron-5
- carbon-11
- boron-11
If we assume that pink represents protons, and green represents neutrons, which nucleus does not represent one of the isotopes of hydrogen?
-
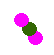
-
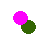
-

-

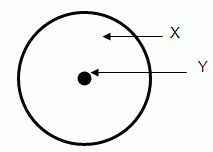
Which of the following is/are true of the region of the atom labeled "X":

Which of the following is/are true of the region of the atom labeled "Y"
Beta decay is generally represented by the symbol _________ on the product side of the equation.
- 42He
- 0-1He
- 0-1e
- 00γ
Alpha decay is generally represented by the symbol _________ on the product side of the equation.
- 42He
- 0-1He
- 0-1e
- 00γ
Gamma decay is generally represented by the symbol _________ on the product side of the equation.
- 42He
- 01e
- 0-1e
- 11γ
Identify the missing particle in the following nuclear reaction:
23993Np → 23994Pu + _____
- 10n
- 01e
- 0-1e
- 11H
What type of decay is taking place in the nuclear reaction shown below?
23993Np → 23994Pu + 0-1e
Which of the following statements best describes the changes occuring in the reaction below?
23993Np → 23994Pu + 0-1e
- a proton has been converted to an electron
- a proton has been converted to a neutron
- a neutron has been converted to an electron
- a neutron has been converted to a proton
Identify the missing particle in the following nuclear reaction:
10n + 23592U → 2 10n + _____ + 13752Te + 00γ
- 42He
- 9738Sr
- 9640Zr
- 9740Zr
What type of decay is evident in the nuclear reaction shown below?
10n + 23592U → 2 10n + 9740Zr + 13752Te + 00γ
Which of the following statements best describes the changes occuring in the reaction below?
10n + 23592U → 2 10n +9740Zr + 13752Te + 00γ
- uranium is undergoing nuclear fission
- uranium is converted to neutrons and energy
- uranium has been converted to zirconium
- zirconium has been converted to neutrons
Identify the missing particle in the following nuclear reaction:
23992U + 42He → _____ + 10n
- 24294Pu
- 24394Pu
- 24392U
- 24192U
What type of particle is evident in the nuclear reaction shown below?
23992U + 42He → 24294Pu + 10n
Which of the following statements best describes the changes occuring in the reaction below?
23992U + 42He → 24294Pu + 10n
- uranium is being converted to plutonium
- helium is being converted to neutrons
- helium is being converted to plutonium
- nuclear fission is taking place
Identify the missing particle in the following nuclear reaction:
2713Al + _____ → 2411Na + 42He
- 10n
- 10H
- 0-1e
- 2813Al
What type of decay is evident in the nuclear reaction shown below?
2713Al + 10n → 2411Na + 42He
Which of the following statements best describes the changes occuring in the reaction below?
2713Al + 10n → 2411Na + 42He
- aluminum decays into sodium
- a neutron becomes an alpha particle
- aluminum decays into a neutron
- aluminum produces a beta particle
Identify the missing particle in the following nuclear reaction:
94Be + 11H → _____ + 42He
- 63Li
- 147N
- 52He
- 41H
What type of decay is evident in the nuclear reaction shown below?
94Be + 11H → 63Li + 42He
Identify the missing particle in the following nuclear reaction:
_____ → 42He + 20881Tl + 00γ
- 21283Bi
- 20479Au
- 20483Bi
- 21279Au
What type of decay is evident in the nuclear reaction shown below?
21283Bi → 42He + 20881Tl + 00γ
Which of the following statements best describes the changes occuring in the reaction below?
21283Bi → 42He + 20881Tl + 00γ
- bismuth is converted to thallium
- bismuth is converted to thulium
- bismuth is converted to helium
- helium produces thallium
Identify the missing particle in the following nuclear reaction:
146C → 0-1e + _____
- 147N
- 145B
- 146C
- 157N
What type of decay is evident in the nuclear reaction shown below?
146C → 0-1e + 147N
Which of the following statements best describes the changes occuring in the reaction below?
146C → 0-1e + 147N
- carbon-14 decays to become nitrogen-14
- one isotope of carbon becomes another isotope of carbon
- carbon turns into an electron
- nitrogen decays to form an electron
Which of the following is the correct orbital notation for the 3d sublevel of the element nickel, (Ni, atomic #28).
-
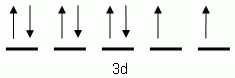
-
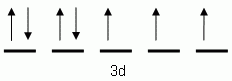
-
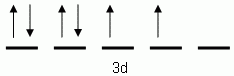
-
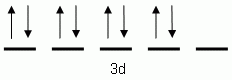
What element has the electron configuration notation 1s22s22p63s1?
Which of the following is the correct orbital notation for the element carbon, (C, atomic #6)?
-
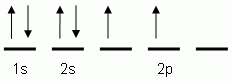
-
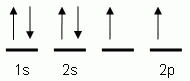
-
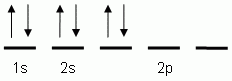
-
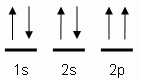
What element has the configuration notation 1s22s22p4?
- Neon
- Oxygen
- Carbon
- Nitrogen
What element has the configuration notation 1s22s22p63s23p5?
- sulfur
- phosphorus
- argon
- chlorine
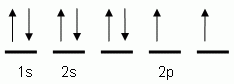
Which of the following is the correct orbital notation for the element oxygen (O, atomic #8)?
-
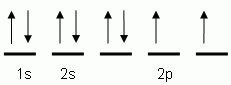
-
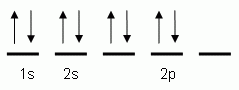
-
-
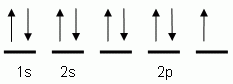
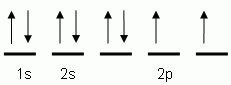
The "up" and "down" arrows in electron orbital notation, such as are shown here, depict:
- electrons and protons attracting each other
- electrons with opposite spins
- chemical bonds
- electrons attracting each other
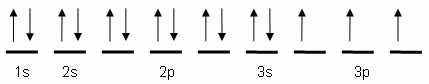
The orbital notation shown is for the element