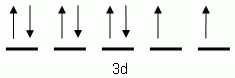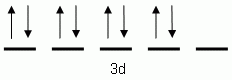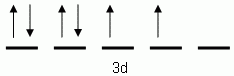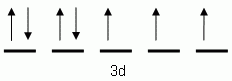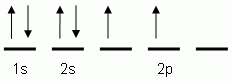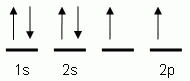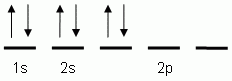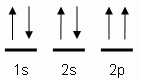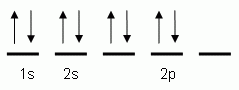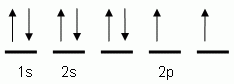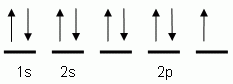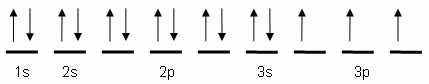
The orbital notation depicted here is for the element:- Which of the following is the correct orbital notation for the 3d sublevel of the element nickel, (Ni, atomic #28).
-
- What element has the electron configuration notation 1s22s22p63s1?
- Which of the following is the correct configuration notation for the element titanium (Ti, atomic number 22)?
- 1s22s22p63s23p63d2
- 1s22s22p63s23p64s24d2
- 1s22s22p63s23p64s23d2
- 1s22s22p63s23p64s21d2
- (This question would be extra credit) The electron configuration for the element bismuth, (Bi, atomic #83) is:
- 1s22s22p63s23p64s24d104p65s25d105p66s26f146d106p3
- 1s22s22p63s23p64s23d104p65s24d105p66s25d106p3
- 1s22s22p63s23p64s24d104p65s25d105p66s26d106p3
- 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p3
- The noble-gas notation for the element indium, (In, atomic #49) is:
- [Kr]5s24d105p1
- [Kr]5s25d105p1
- [Kr]5p1
- [Kr]5s24f145d105p1
- Which of the following is the correct orbital notation for the element carbon, (C, atomic #6)?
-
- What element has the noble-gas notation [Ne]3s23p5?
- Sulfur
- Phosphorus
- Argon
- Chlorine
- What element has the noble-gas notation [Xe]6s2?
- What element has the configuration notation 1s22s22p4?
- Neon
- Oxygen
- Nitrogen
- Fluorine
- Which of the following is the correct electron configuration notation for the element nitrogen, (N, atomic # 7)?
- 1s22s22p3
- 1s21p5
- 1s22s23p3
- 1s22s23s24s1
- Which of the following is the correct orbital notation for the element oxygen (O, atomic #8)?
-
- Which of the following is the correct noble-gas notation for the element strontium (Sr, atomic #38)?
- [Kr]5s1
- [Kr]6s2
- [Kr]5s2
- [Xe]5s2
- What element has the noble gas configuration [Ne]3s23p1?
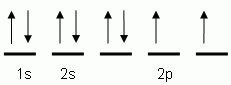
The "up" and "down" arrows in electron orbital notation, such as are shown here, depict:- electrons and protons attracting each other
- electrons with opposite spins
- oppositely charged electrons
- protons and neutrons in orbitals