- The nucleus of most atoms is made up of:
- protons and electrons
- protons and neutrons
- neutrons and electrons
- electrons and protons
- The charge and mass number of an electron are:
- charge = +1, Mass number = 0
- charge = -1, Mass number = 0
- charge = 0, Mass number = 1
- charge = +1, Mass number = 1
- The charge and mass number of a proton are:
- charge = +1, Mass number = 0
- charge = -1, Mass number = 0
- charge = 0, Mass number = 1
- charge = +1, Mass number = 1
- The charge and mass number of a neutron are:
- charge = +1, Mass number = 0
- charge = -1, Mass number = 0
- charge = 0, Mass number = 1
- charge = +1, Mass number = 1
- Most of the mass of the atom can be found in the:
- electron cloud
- nucleus
- electrons
- charges
- The atomic number of an element is equal to:
- the number of protons in the atom
- the number of neutrons in the atom
- the number of protons plus the number of neutrons
- the number of protons plus the number of electrons
- The mass number of an atom is determined by:
- adding the protons and electrons
- adding the neutrons and electrons
- adding the neutrons and protons
- the number of protons only
- The laws of electrostatics consistently demonstrate that opposite charges:
- destroy one another
- attract
- repel
- The laws of electrostatics consistently demonstrate that "like" (identical) charges:
- destroy one another
- attract
- repel
- The _______________ constitute(s) most of the volume of an atom.
- protons
- neutrons
- nucleus
- electron cloud
- Which of the following descriptions apply to the nucleus?
- Of the basic atomic particles, the one that would be attracted to a negatively charged metallic plate is the:
- proton
- neutron
- electron
- The nucleus of the atom is held together by:
- electrons
- nuclear forces
- magnetism
- electrostatic attraction
- Isotopes (such as hydrogen-1, hydrogen-2, and hydrogen-3) are atoms of the same element that differ in:
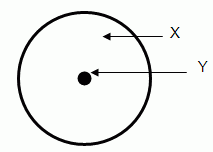
The letter "X" in the diagram above marks the:
The letter "Y" in the diagram above marks the:
The region labeled "X" in the diagram has a charge that is:- positive
- negative
- neutral

The region labeled "Y" in the diagram has a charge that is:- positive
- negative
- neutral