- In all electrochemical cells, the process that takes place at the anode is _________________ and the process that takes place at the cathode is _________________.
- reduction, oxidation
- oxidation, reduction
- reduction, reduction
- oxidation, oxidation
- The standard hydrogen electrode is assigned a potential of:
- zero volts
- 1.00 volts
- -1.00 volts
- 0.76 volts
- Calculate the value of E0cell for a galvanic cell that contains the following half cells:
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
- Cu+ + e- → Cu (E0 = 0.52v)
- E0cell = 0.32 volts
- E0cell = 0.84 volts
- E0cell = -0.32 volts
- E0cell = -0.84 volts
- E0cell = 1.88 volts
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
- For the galvanic cell described below, the correct line notation is:
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
- Cu+ + e- → Cu (E0 = 0.52v)
- Cu(s)|Cu+(aq)||Cl2(g)|2Cl-(aq)
- Pt(s)|Cu(s)|Cu+(aq)||Cl2(g)|2Cl-(aq)|Pt(s)
- Cu(s)|Cu+(aq)||Cl2(g)|2Cl-(aq)|Pt(s)
- Pt(s)|Cl2(g)|2Cl-(aq)||Cu(s)|Cu+(aq)|Pt(s)
- Pt(s)|Cl2(g)|2Cl-(aq)||Cu(s)|Cu+(aq)
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
- Calculate DG0 for the reaction:
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
- Cu+ + e- → Cu (E0 = 0.52v)
- -8.1 x 104 J
- 8.1 x 105 J
- -3.1 x 104 J
- -6.2 x 104 J
- -1.6 x 105 J
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
- The equilibrium constant (K) for the reaction below is approximately:
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
- Cu+ + e- → Cu (E0 = 0.52v)
- 1028
- 1017
- 102
- 10-34
- 1011
- Cl2 + 2e- → 2Cl- (E0 = 1.36v)
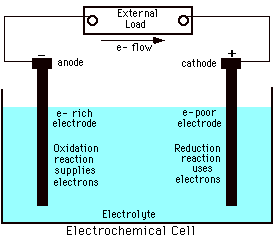
This picture of an electrochemical cell can best be described as:- a complete electrolytic cell
- an electrolytic cell, but missing at least one essential component
- a complete galvanic cell
- a galvanic cell, but missing at least one essential component
- Reduction potential is:
- always positive
- an intensive property
- an extensive property
- measured in amperes
- A mole of electrons has a charge of 96,485 coulombs per mole of electrons. This quantity is known to chemists as:
- 1 ampere
- 1 volt
- 1 joule
- 1 faraday
- 1 watt
- What is the oxidation state of S in H2SO3?
- +6
- +4
- +2
- 0
- -3
- The equation that represents a reaction that is not a redox reaction is:
1. 2H2 + O2 → 2H2O
2. Zn + CuSO4 → ZnSO4 + Cu
3. 2H2O2 → 2H2O + O2
4. H2O + CO2 → H2CO3- Reaction 1
- Reaction 2
- Reaction 3
- Reaction 4
- All of these are redox reactions
- How many electrons are transferred in the following reaction?
2NaCl + 2H2SO4 + MnO2 → Na2SO4 + MnSO4 + 2H2O + Cl2- 1
- 2
- 4
- 6
- 8
- In the reaction below, the substance that is reduced is:
2NaCl + 2H2SO4 + MnO2 → Na2SO4 + MnSO4 + 2H2O + Cl2- Na+
- Cl-
- H+
- Mn4+
- O2-
- Which of the following would be most easily oxidized?
- Na
- Na+
- F-
- Cl2
- O2-
- In the electrolytic decomposition of water:
2H2O → 2H2 + O2- hydrogen is formed at the cathode and oxygen is formed at the anode
- hydrogen is formed at the anode and oxygen is formed at the cathode
- hydrogen is formed at both the anode and the cathode
- oxygen is formed at both the anode and the cathode
- What value of E do you expect for
Pb(s) → Pb2+ + 2e-
in 0.015m Pb2+ solution? E0 = +0.13v- E = +0.13v
- E = +0.18v
- E = +0.83v
- E = +0.27v
- E = 0.00v
- The current in a given wire is 1.80 amp. How many coulombs will pass a given point on the wire in 1.36 minutes?
- 2.45 C
- 1.32 C
- 45.3 C
- 147 C
- 253 C
- Two cells, one containing aqueous AgNO3 and the other containing CuSO4 are set up in series. In a given electrolysis that results in depositing 1.25 g of silver in the first cell, how much copper should deposit simultaneously in the second cell?
- 1.25 g
- 0.736 g
- 0.368 g
- 1.47 g
- 2.65 g
- With a current of 20.0 amps, how long would it take to generate 1.00 kilograms of aluminum by the reaction:
Al3+ + 3e- → Al(s)- 5.36 x 105 sec
- 1.78 x 105 sec
- 2.19 x 104 sec
- 3.90 x 103 sec
- 1.68 x 102 sec
- If a constant current of 8.00 amperes is passed through a cell containing Zn2+ for 2.00 hours, how many grams of zinc will plate out onto the cathode?
- 39.0 g
- 19.5 g
- 0.985 g
- 126 g
- 1.43 x 103 g