- Which of the following pairs of compounds could create a suitable buffer system?
- sodium chloride and sodium hydroxide
- sodium sulfate and sodium hydroxide
- sodium acetate and acetic acid
- carbonic acid and sodium chloride
- ammonia and potassium hydroxide
- What is the pH of a solution that is 0.50 M in propanoic acid and 0.40 M in sodium propanoate. (Ka for propanoic acid = 1.3 x 10-5)
- 0.097
- 4.98
- 0.47
- 4.79
- -4.98
- A buffer solution is prepared that is 0.50 M in propanoic acid and 0.40 M in sodium propanoate with a solution volume of 1.00 liters. (Ka for propanoic acid = 1.3 x 10-5). What is the pH of the solution when 0.040 mol of HCl(g) is added to the solution? Assume no change in solution volume.
- 4.97
- 4.67
- 4.91
- 4.71
- 4.77
- A buffer solution is prepared that is 0.50 M in propanoic acid and 0.40 M in sodium propanoate with a solution volume of 1.00 liters. (Ka for propanoic acid = 1.3 x 10-5). What is the pH of the solution when 0.060 mol of NaOH(s) is added to the solution? Assume no change in solution volume.
- 3.97
- 4.67
- 4.77
- 4.91
- 4.97
- Suppose that 0.250 liters of a buffer solution that contains 0.225 M acetic acid and 0.225 M sodium acetate. What would be the pH change if 30.0 mL of 0.100 M HCl is added to this buffer? Assume volumes are additive. Ka for acetic acid is 1.8 x 10 -5.
- 4.70 to 4.78
- 4.74 to 4.70
- 4.74 to 4.82
- 5.40 to 7.68
- 6.40 to 4.56
- What is the pH of a buffer solution consisting of 0.150 mol of NH3 plus 0.250 mol of NH4Cl in enough water to make 0.750 liter of solution. Kb = 1.81 x 10-5.
- 9.036
- 4.963
- 2.721
- 11.279
- 8.547
- 80.0 mL of a buffer solution contains 0.169 M NH3 and 0.183 M NH4Cl. If you add 10.0 mL of 0.100 M HCl, what will be the pH? Kb = 1.81 x 10-5. Assume additive volumes.
- 9.161
- 4.963
- 2.721
- 11.279
- 7.908
- Ka for HNO2 is 4.5 x 10-4, calculate the pH of a buffer solution made by mixing 0.225 mol of HNO2 and 0.450 mol of NaNO2 in enough water to make 0.400 liter of solution.
- 3.51
- 3.65
- 3.90
- 5.72
- 2.98
- Suppose you are given 0.50 liter of 0.500 M acetic acid, and 0.50 liter of 0.250 M sodium acetate. What is the maximum volume of buffer solution that you can make if the buffer must have a pH of 4.58? Ka for acetic acid is 1.8 x 10-5.
- 0.50 liter
- 0.287 liter
- 0.91 liter
- 0.87 liter
- 0.78 liter
- A chemist desires to create a buffer solution beginning with 1.00 liter of 0.200 M NH3. How many moles of gaseous HCl must be introduced in order to produce a buffer of maximum capacity. Assume no increase in solution volume.
- 0.100 mol HCl
- 0.200 mol HCl
- 0.300 mol HCl
- 0.500 mol HCl
- None - the solution is already buffered
- The Ksp of BaSO4 is 1.5 x 10-9. What is the molar concentration of Ba2+(aq) in a saturated solution of BaSO4?
- 2.25 x 10-18 M
- 2.45 x 10-5 M
- 6.7 x 10-15 M
- 3.9 x 10-5 M
- 1.7 x 10-2 M
- The Ksp of BaSO4 is 1.5 x 10-9. How many grams of BaSO4 can be dissolved in 1000. liters of solution?
- 0.085 g
- 170 g
- 9.0 g
- 2.3 g
- 39 g
- Calculate the Ksp of Ba(OH)2 given the fact that the solubility of Ba(OH)2 in water is 4.6 g per 0.250 liter.
- 5.0 x 10-3
- 1.2 x 10-2
- 1.1 x 10-1
- 2.0 x 10-7
- 1.5 x 10-4
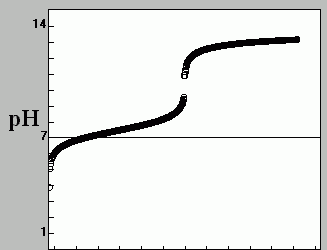
The image above shows the titration of a:- strong acid with a strong base
- Strong acid with a weak base
- Weak acid with a strong acid
- Weak acid with a strong base
- weak base with a strong base
- 200.0 mL of 0.200 M HCl is titrated with 0.050 M NaOH. What is the pH after the addition of 100. mL of the NaOH solution?
- 1.45
- 0.76
- 0.93
- 1.03
- 0.82
- 50.0 mL of 0.100 M NH3 is titrated with 0.025 M HCl. What is the pH of the solution after 100.0 mL of HCl has been added? Kb for NH3 1.81 x 10-5.
- 4.74
- 9.26
- 12.45
- 8.99
- 10.90