- The only intermolecular forces existing between oxygen molecules are:
- permanent dipole forces
- hydrogen bonding forces
- London dispersion forces
- ion-ion attractive forces
- nuclear forces
- Which of the following substances will have the greatest vapor pressure at 0°C?
- NaCl
- H2O
- NH3
- CH4
- H2O and NH3
- Place the following in order of increasing strength:
1. hydrogen bonding
2. covalent bonding
3. London dispersion forces- 1, 2, 3
- 3, 2, 1
- 1, 3, 2
- 2, 1, 3
- 3, 1, 2
- Which of the following hydrocarbons has the highest boiling point?
- CH4
- C2H6
- C3H8
- C4H10
- These are all gases and do not boil
- A metal has crystallized with a face-centered cubic lattice. The edge of the unit cell is 366 pm. What is the diameter of the metal atom?
- 518 pm
- 259 pm
- 267912 pm
- 0.0296 pm
- 3.98 x 10-5 pm
- For a solution in a closed container at equilibrium, which of the following pairs represent processes that must be occuring at equal rates?
- evaporation and crystallization
- fusion and boiling
- evaporation and sublimation
- crystallization and sublimation
- evaporation and condensation
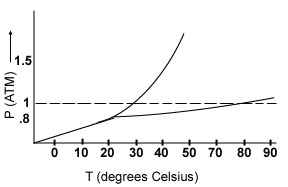
The normal boiling point of X is most likely:- 22°C
- 28°C
- 50°C
- 80°C
- 90°C

At a pressure of 1.3 atm and 20°C, compound X exists as:- a solid only
- a solid and a liquid
- a liquid and a gas
- a solid and a gas
- a liquid only

The triple point of compound X occurs at a temperature of:- -10°C
- 23°C
- 50°C
- 80°C
- 29°C

As the pressure increases, the melting point of compound X will:- increase
- decrease
- stay the same
- increase and then decrease
- decrease and then increase
- An example of an amorphous solid is:
- ice
- quartz
- plastic
- diamond
- gold
- Buckminsterfullerene is one of the allotropic forms of:
- silicon
- semimetals
- oxygen
- carbon
- steel
- Which of the following statements is false?
1. Liquids with large intermolecular forces tend to have very low boiling points
2. Liquids with large intermolecular forces tend to have considerable surface tension
3. When a substance changes from a solid to a liquid, the molecules remain intact
4. The hardness of diamond is due to strong dipole-dipole attraction- 1 and 3
- 2 and 4
- 3 and 4
- 1 and 4
- 2 and 3
- Which of the following is not an endothermic process?
- crystallization
- vaporization
- melting
- sublimation
- combustion
- How much energy is needed to convert 50.0 g of ice at -5.00°C to water at 25°C?
- Specific heat(ice) = 2.10 J/(g°C)
- Specific heat(water) = 4.18 J/(g°C)
- Heat of fusion = 333 J/g
- Heat of vaporization = 2258 J/g
- 22.4 kJ
- 21.9 kJ
- 37.3 kJ
- 175 J
- 18.0 J
- Specific heat(ice) = 2.10 J/(g°C)
- The correct order for increasing boiling point among the noble gases is:
1. He
2. Ne
3. Ar
4. Kr
5. Xe
This phenomenon is best explained in terms of:- hydrogen bonding
- dipole-dipole interaction
- covalent bonding
- Hund's rule
- London dispersion forces
- The triple point of CO2 is 5.2 atm and -57°C. In a laboratory at EDHS, on a day when the pressure is 758 torr and the temperature in the room is 20°C, a solid sample of carbon dioxide will:
- freeze
- boil
- melt
- remain solid
- sublime