- The geometry associated with sp3 is:
- linear
- octahedral
- trigonal planar
- tetrahedral
- trigonal bipyramid
- What is the hybridization of the carbon in CO2?
- sp
- sp2
- sp3
- dsp3
- d2sp3
- The bond angle between C - Cl bonds in carbon tetrachloride, CCl4 is expected to be:
- 180°
- 90°
- 109.5°
- 120°
- 60°
- How many pi (p) bonds does the molecule methanol (CH3OH) contain?
- none
- one
- two
- three
- four
- Double bonds can be thought of as consisting of:
- Two sigma (s) bonds
- Two pi (p) bonds
- One sigma (s) bond
- One pi (p) bond
- A sigma (s) bond and a pi (p) bond
- Hybridization of carbon in the molecule CH2O is of the form
- sp
- sp2
- sp3
- dsp3
- d2sp3
- The geometry associated with d2sp3 hybridization is:
- linear
- octahedral
- tetrahedral
- trigonal planar
- trigonal biplanar
- The geometry associated with sp2 hybridization is:
- linear
- octahedral
- tetrahedral
- trigonal planar
- trigonal biplanar
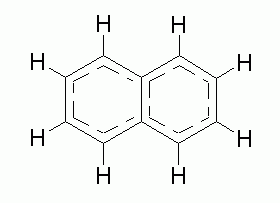
The hybridization around each carbon in naphthalene is:- sp
- sp2
- sp3
- dsp3
- d2sp3

How many sigma (s) bonds are present in one molecule of naphthalene?- 6
- 8
- 11
- 17
- 19

How many pi (p) bonds are present in one molecule of naphthalene?- none
- one
- three
- five
- seven
- How many electrons are involved in pi (p) bonding in a molecule of ethyne (acetylene) C2H2?
- none
- one
- two
- three
- four
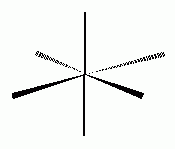
The type of hybridization associated with the geometry shown above is:- sp
- sp2
- sp3
- dsp3
- d2sp3
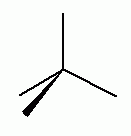
The type of hybridization associated with the geometry shown above is:- sp
- sp2
- sp3
- dsp3
- d2sp3
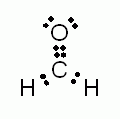
How many sigma (s) bonds are present in the molecule depicted above?- 1
- 2
- 3
- 4
- 5

How many pi (p) bonds are present in the molecule depicted above?- 1
- 2
- 3
- 4
- 5