- In which of the following compounds does the bond between the central atom and chlorine have the greatest ionic character?
- CaCl2
- CCl4
- HCl
- BCl3
- FeCl2
- Which of the following is the largest ion?
- Li+
- Na+
- K+
- Rb+
- Cs+
- Which of the following compounds is most likely to be ionic?
- CO2
- CCl4
- MgCl2
- HBr
- FeCl3
- Which of the following bonds is the least polar?
- H - O
- C - Cl
- B - F
- Na - O
- Al - S
- Which of the following molecules has a dipole moment?
- H2O
- CCl4
- CO2
- CH4
- Br2
- Which of these is an isoelectronic series?
- Li, Na, K
- P3-, S2-, Cl-
- O, S, Se
- K+, Ca2+, Ga3+
- Li+, Na+, K+,
- Which statement best describes the bond angles in NH3?
- Exactly 120°
- Exactly 180°
- Approximately 109°
- Slightly less than 109°
- Slightly less than 90°
- Given the following bond energies, estimate the enthalpy change (heat of reaction) for the combustion of methane (CH4).
- C - C, 347 kJ/mol
- C = C, 614 kJ/mol
- C - O, 358 kJ/mol
- C = O, 799 kJ/mol
- C - H, 413 kJ/mol
- O - H, 463 kJ/mol
- O - O, 146 kJ/mol
- O = O, 495 kJ/mol
- 74 kJ
- -245 kJ
- -74 kJ
- -808 kJ
- 3132 kJ
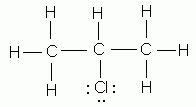
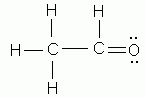
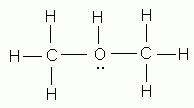
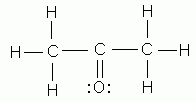
- As indicated by Lewis structures, which of the following would probably not exist as a stable molecule?
- CH3Cl
- CH3OH
- C2H5OH
- C2O3
- CH2Cl2
- Which of these Lewis structures is invalid?
-
- Which of the following molecules must contain at least one double bond
- CH3I
- CH3COOH
- H2O2
- CCl4
- H2O
- The Lewis structure for hydrogen cyanide is:
-
- How many unshared electron pairs must be included in the Lewis structure for water, H2O?
- 0
- 1
- 2
- 3
- 4
- How many of the following molecules can have their structures drawn within a plane?
- CH4
- H2O
- CO2
- BF3
- all
- one
- two
- three
- none
- In the Lewis structure for CH2Cl2, the central atom shares __________ electrons.
- 2
- 4
- 6
- 8
- 10
- In the Lewis structure for CH2Cl2, the number of unshared electron pairs is:
- 2
- 4
- 6
- 8
- 10
- Which of the following does not describe a molecule with zero net dipole moment
- Diatomic molecule of an element
- Trigonal planar molecule, three identical bonds
- Tetrahedral molecule, 4 identical polar bonds
- Linear molecule of a hydrogen halide
- All of these have zero net dipole moment
- Nitrogen triiodide, NI3, is an unstable molecule that is used as a contact explosive. Its molecular structure is:
- pyramidal
- tetrahedral
- square planar
- octahedral
- none of these
- Which of the following statements is false?
- Within the representative elements, electronegativity tends to increase with decreasing atomic radius.
- Cations tend to be smaller than the corresponding atom from which they were formed.
- High electronegativity is characteristic of nonmetals.
- Semi-metals have generally higher electronegativity than metals.
- All of these statements are true.
- Which of the following ionic compounds has the smallest lattice energy?
- LiF
- CaCl2
- Na2O
- CaO
- MgS