- How many of the following salts can be expected to be insoluble in water?
- sodium sulfate
- potassium chloride
- ammonium nitrate
- potassium chromate
- none
- one
- two
- three
- four
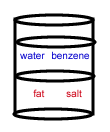
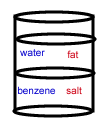
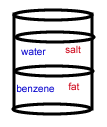
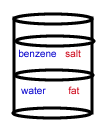
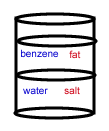
- A chemist wants to separate a mixture of salt and fat. He places the mixture in a container with the nonpolar solvent benzene (density = 0.8787 g/cm3) and water (density = 1.00 g/cm3). After shaking the solution and allowing it to settle, the correct arrangement of solvent and solute is:
-
- A 25.0-g sample of sodium hydroxide is dissolved in 400. mL of water. What is the concentration of the solution?
- 62.5 M
- 0.10 M
- 1.56 M
- 1.56 x 10-3 M
- 100. M
- How many grams of potassium nitrate are required to prepare 3.00 x 102 mL of 0.750 M solution?
- 0.00223 g
- 2.28 x 104 g
- 2.4 g
- 22.8 g
- 84.5 g
- How many grams of sodium chloride are dissolved in 50.0 mL of 1.50 M solution?
- 4.38 g
- 4.38 x 103 g
- 23.4 g
- 117 g
- 0.00324 g
- A chemist makes a stock solution of potassium chromate solution by dissolving 97.1 grams of the compound in 1.00 liter of solution. What volume of the solution must be diluted with water in order to prepare 200. mL of 0.200 M solution?
- 0.0800 mL
- 80.0 mL
- 120. mL
- 0.150 L
- 750. mL
- A 500 mL sample of a 0.350 M solution is left open on a lab counter for twl weeks, after which the concentration of the solution is 0.955 M. What is the new volume of the solution?
- 223 mL
- 183 L
- 0.605 L
- 1.83 mL
- 0.183 L
- All of the following are weak acids except:
- HNO3
- HF
- H2CO3
- H2S
- HClO
- In a reaction between ammonium phosphate and silver nitrate in aqueous solution, which of the following terms will be present in the balanced molecular equation?
- NH4PO4(aq)
- AgNO3(s)
- 3NH4NO3(aq)
- Ag3PO4(aq)
- None of these
- When solutions of sodium nitrate and potassium chloride are mixed, which of the following will be present in the net ionic equation?
- Na+
- KNO3(s)
- Cl-
- NaCl(aq)
- None of these
- Which of the following is the most soluble base?
- Ba(OH)2
- NaOH
- Ca(OH)2
- Mg(OH)2
- Al(OH)3
- Which of the following ions is most likely to combine with an anion to form an insoluble salt?
- sodium
- sulfide
- silver
- potassium
- ammonium
- In the balanced molecular equation for the neutralization reaction between phosphoric acid and potassium hydroxide, the products are:
- KPO4 + H3OH
- K3PO4 + 3H2O
- K+(aq) + PO43-(aq) + 3H+ + 3OH-
- 3H2O
- None of these
- In which of the following compounds does sulfur have an oxidation state of +4?
- H2SO4
- H2SO3
- H2S
- SO3
- MgSO4
- What volume of 0.250 M H2SO4 is needed to react completely with 14.5 grams of NaOH?
- 1.45 L
- 2.90 L
- 0.237 mL
- 11.0 L
- 0.726 L
- 435 mL of a 0.100 M HNO3 solution is mixed with 235 mL of 0.175 M Ca(OH)2. Which of the following statements are true?
1. The solution is alkaline
2. The solution is acidic
3. Water is a product
4. Some HNO3 remains unreacted
5. Some Ca(OH)2 remains unreacted- 1 and 5
- 3 and 4
- 2 and 3
- 1 and 4
- 1, 3 and 5
- In the reaction 4Al + 3O2 → 2Al2O3 the substance oxidized is:
- Al
- O2
- Al3+
- O2-
- None of these, because this is not a redox reaction
- NaCl + H2SO4 + MnO2 → Na2SO4 + MnSO4 + H2O + Cl2
In this reaction, the ____ ion is oxidized.- Na+
- Cl-
- O2-
- H+
- Mn4+
- In the following reaction, the oxidation numbers on sulfur are:
NaCl + H2SO4 + MnO2 → Na2SO4 + MnSO4 + H2O + Cl2- +6, +6 and +4 respectively
- +4, +6 and +4 respectively
- +4, +4 and +4 respectively
- +6, +6 and +6 respectively
- +4, +6 and +6 respectively
- When the following reaction is balanced, the sum of all of the coefficients in the equation is:
NaCl + H2SO4 + MnO2 → Na2SO4 + MnSO4 + H2O + Cl2- 6
- 10
- 11
- 14
- 16