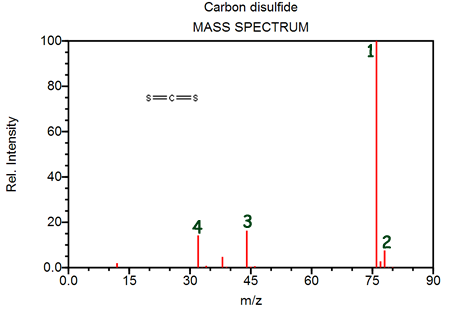
Peak 1 is primarily due to- [12C32S2]+
- [14C30S32S]+
- [12C30S34S]+
- [12C34S2]+

Peak 2 corresponds to- [14C32S2]+
- [14C30S32S]+
- [12C30S34S]+
- [12C34S2]+

Peak 3 corresponds to- [14C32S2]+
- [12C34S]+
- [12C32S]+
- [14C30S]+

Peak 4 provides evidence of- carbon-12
- [34S]+
- [32S]+
- [30S]+
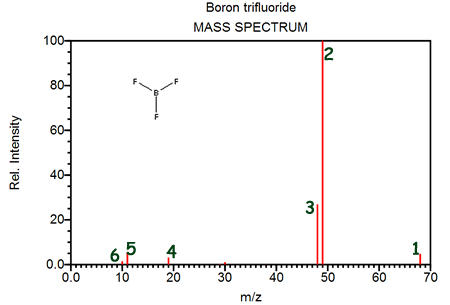
The diagram shown here provides evidence for the existence of the TWO isotopes- boron-10 and boron-11
- fluorine-19 and fluorine-20
- boron-11 and fluorine-20
- boron-9 and boron-12

Peak 1 identifies- [11B19F3]+
- fluorine-19 and fluorine-20
- boron-11 and fluorine-20
- boron-9 and boron-12

The difference between peaks 2 and 3 is- The fragment at peak 2 contains boron-11 and the fragment at peak 3 contains boron-10
- The fragment at peak 3 contains three atoms of fluorine and the fragment at peak 2 contains only two atoms of fluorine
- The fragment at peak 3 contains boron-11 and the fragment at peak 2 contains fluorine-18
- The fragment at peak 3 contains an atom of hydrogen not contained in the fragment at peak 2
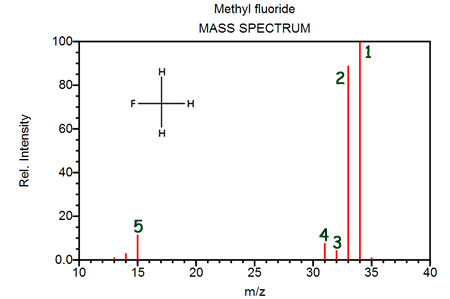
The peak labeled "1" corresponds to- [1H312C19F]+
- The fragment at peak 3 contains three atoms of fluorine and the fragment at peak 2 contains only two atoms of fluorine
- The fragment at peak 3 contains boron-11 and the fragment at peak 2 contains fluorine-18
- The fragment at peak 3 contains an atom of hydrogen not contained in the fragment at peak 2

The masses of peaks 1, 2, 3, and 4 are successively smaller in mass by one. This can be explained by- Successively fewer atoms of hydrogen-1
- different isotopes of carbon
- different isotopes of carbon and hydrogen
- different isotopes of fluorine

The peak labeled "5" corresponds primarily to- [1H312C]+
- [3H12C]+
- [2H211C]+
- [12C19F]+

There is a barely noticeable peak at a mass = 35. The best explanation for this is- [1H313C19F]+
- [1H312C20F]+
- [2H1H212C19F]+
- [2H14C19F]+
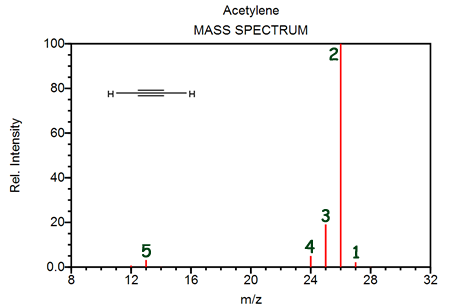
The peak labeled "2" identifies primarily- [1H212C2]+
- [1H312C20F]+
- [2H1H212C19F]+
- [2H14C19F]+

The masses of peaks 2, 3, and 4 are successively smaller in mass by one. This can be explained by- successively fewer atoms of hydrogen-1
- the isotopes of carbon (carbon-12, carbon-13 and carbon-14)
- the largest peak containing hydrogen-3, the next largest containing hydrogen-2, and the smallest containing hydrogen-1
- contamination of the sample tested
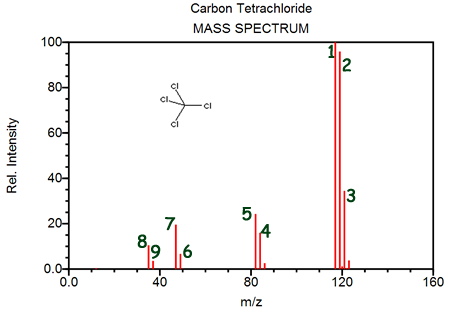
Which of the following is NOT represented on this diagram?- [12C35Cl4]+
- the isotopes of carbon (carbon-12, carbon-13 and carbon-14)
- the largest peak containing hydrogen-3, the next largest containing hydrogen-2, and the smallest containing hydrogen-1
- contamination of the sample tested

Peak #3 represents- [12C35Cl4]+
- [12C35Cl3]+
- [12C35Cl237Cl]+
- [12C35Cl37Cl2]+

Peaks 8 and 9 are evidence of- two isotopes of carbon
- chlorine's existence as a diatomic molecule
- two isotopes of chlorine
- chlorine's electron configuration
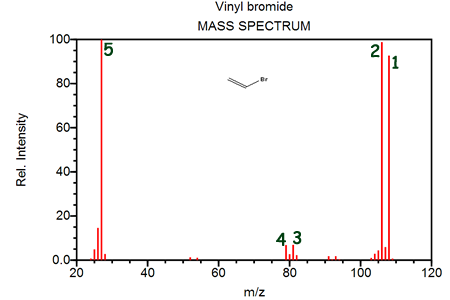
Peak 1 represents primarily- [1H312C281Br]+
- chlorine's existence as a diatomic molecule
- two isotopes of chlorine
- chlorine's electron configuration

Peaks 3 and 4 are evidence for- two isotopes of bromine, of nearly equal abundance
- carbon's ability to form four bonds
- the existence of only one isotope of bromine, having a mass of nearly 80
- the diatomic nature of halogen molecules

The peak labeled "5" represents primarily which fragment?- [1H312C2]+
- [13C12C]+
- [3H11C2]+
- [2H12C2]+

The un-numbered peak with a mass of 25 would be predominantly due to which fragment?- [1H12C2]+
- [13C12C]+
- [3H11C2]+
- [2H12C2]+